Molecular Geometry Of AlCl3 - What Makes Its Shape
Have you ever stopped to think about how tiny chemical building blocks, like the ones that make up everything around us, actually look? It's kind of neat, really, to consider that even the smallest bits of stuff have a particular way of being put together in space. These shapes are not just random; they matter a lot for how these bits act and what they can do. So, too it's almost like understanding their little architectural plans.
When we talk about things like aluminum chloride, which chemists often call AlCl3, its shape is a pretty interesting topic. This particular combination of aluminum and chlorine atoms takes on a certain form, and knowing that form helps us figure out so much more about it. It’s like knowing the blueprint of a small house; you can then picture how people might move inside it, or how it stands up against the wind, you know?
We're going to take a closer look at what gives AlCl3 its particular spatial arrangement. We'll explore the main ideas that help scientists figure out these tiny structures, and we'll see how AlCl3 fits into the bigger picture of how atoms join up. It’s a way to get a better sense of the hidden world of chemistry, actually, and how very important these little shapes are.
- Livvy Dunne Naked Leaks A Comprehensive Exploration Of The Controversy
- Unveiling The World Of Adult Movie Rules A Comprehensive Guide
- Mackenzie Davis Partner A Deep Dive Into Her Relationship
- When Did Aubreigh Wyatt Pass Away A Deep Dive Into Her Life And Legacy
- Understanding The Ages Of Backstreet Boys A Comprehensive Overview
Table of Contents
- What is Molecular Geometry Anyway?
- How Does AlCl3 Get Its Molecular Geometry?
- Figuring Out These Tiny Shapes for Molecular Geometry
- The Role of Electron Pairs in Molecular Geometry
- Does AlCl3 Change Its Molecular Geometry?
- How to Picture AlCl3's Molecular Geometry?
- Why Does AlCl3 Have This Specific Molecular Geometry?
- What Makes AlCl3 Nonpolar in Its Larger Form?
What is Molecular Geometry Anyway?
So, what exactly do we mean when we talk about something having a "molecular" shape? Well, it's pretty simple when you break it down. Think of it this way: a molecule is a collection of atoms that have come together, forming a specific unit. These atoms are the smallest bits of a chemical substance, and when they link up, they do so in a particular three-dimensional pattern. That pattern, the way they are arranged in space, is what we call its molecular geometry. It’s about how these little pieces spread themselves out around a central point, you know?
The idea of something being "molecular" just means it has to do with these tiny groups of atoms. When we say "molecular," we're really talking about something that is made up of, or relates to, these little units. It’s the way things are put together at a very, very small level. For example, if you consider a simple water molecule, it's not just a straight line of atoms; it has a bent shape. That bent shape is its molecular geometry. This particular arrangement of atoms, too, is what gives each substance its unique set of qualities.
Understanding these shapes helps scientists predict how different substances will act when they meet each other. It’s like knowing if a puzzle piece is square or round; that tells you where it might fit. These shapes are determined by the bonds between atoms and the way electrons behave around them. It’s a fundamental idea in chemistry, honestly, giving us a picture of these invisible building blocks.
- Exploring Cardi Bs Father A Deep Dive Into The Life Of Offset
- Understanding The Controversy Surrounding Hunter Bidens Personal Life
- Understanding Vikram Age A Comprehensive Overview
- Shiloh Joliepitt News The Life And Journey Of Angelina Jolie And Brad Pitts Daughter
- Tia Kemp Net Worth 2024 A Comprehensive Analysis
How Does AlCl3 Get Its Molecular Geometry?
Now, let's turn our attention to AlCl3, or aluminum chloride, and how it gets its own particular molecular geometry. This compound is made from one aluminum atom and three chlorine atoms. When these four atoms come together, they don't just clump up any old way. There's a method to their arrangement, and it's quite specific, actually. The central aluminum atom acts like the hub of a wheel, with the three chlorine atoms spreading out around it.
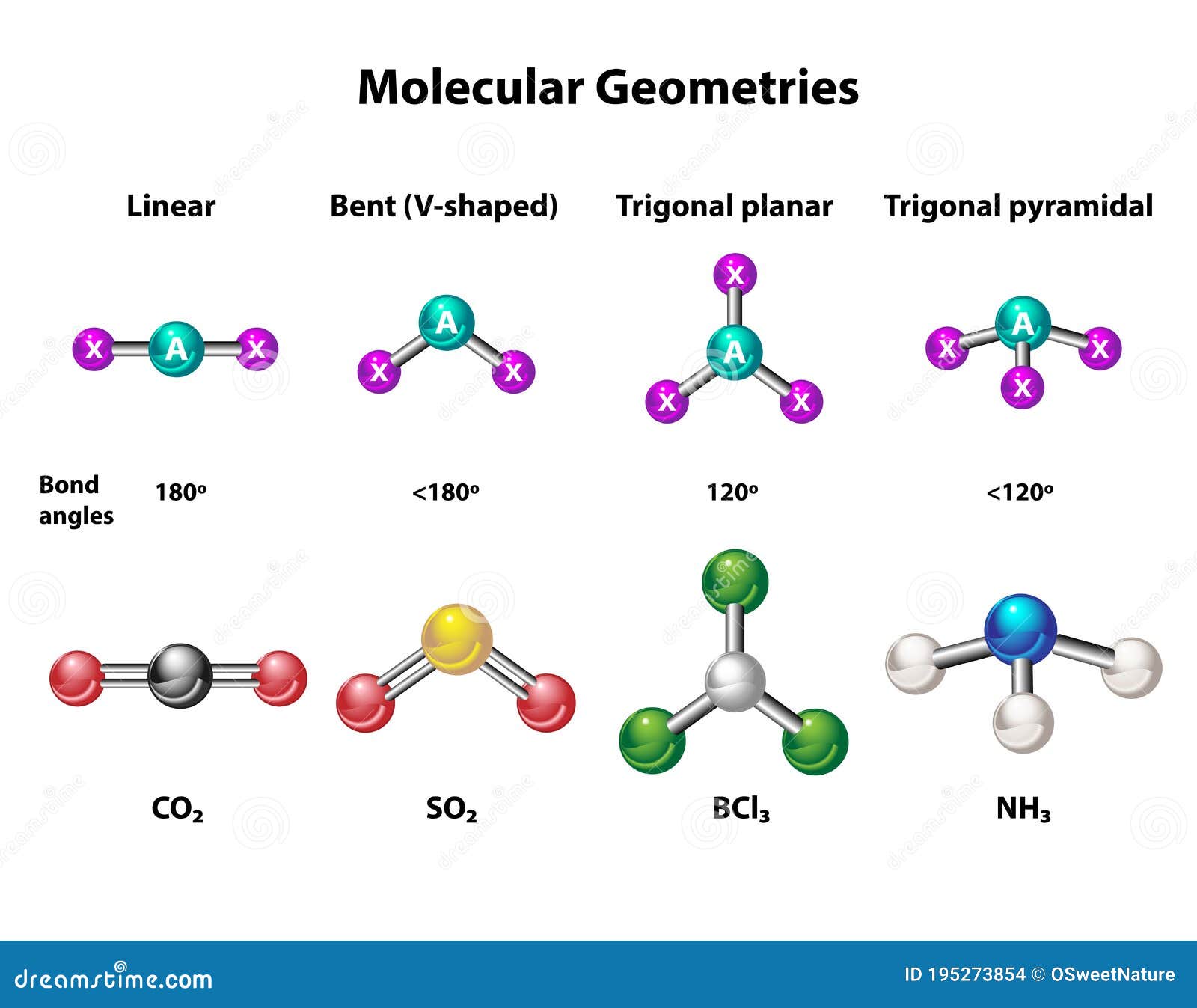
The way these atoms settle into place is largely influenced by something called the Lewis structure. This is a way of drawing out where the electrons are in a molecule, showing how they form bonds. For AlCl3, the Lewis structure gives us a big hint about its shape. It suggests that the molecule will take on what's called a trigonal planar geometry. This means the three chlorine atoms position themselves in a flat, triangular pattern around the aluminum, a bit like the three points of a peace sign, if you can picture that.
This flat, triangular arrangement is a pretty common shape for molecules where a central atom is connected to three other atoms and has no extra, unshared electron pairs on itself. It’s a very balanced way for them to be. The chlorine atoms, you see, are equally spaced around the aluminum, trying to get as far away from each other as they can while still being connected. This natural repulsion between electron groups is what drives the specific molecular geometry of AlCl3.
Figuring Out These Tiny Shapes for Molecular Geometry
So, how do we actually go about figuring out these tiny shapes, like the molecular geometry of something like AlCl3? It's not like we can just look at them with our eyes. Scientists use some clever ideas to predict how atoms will arrange themselves. One of the main tools in this process is something called VSEPR theory. That's short for "Valence Shell Electron Pair Repulsion" theory, and it sounds a bit fancy, but it's really pretty straightforward.
The basic idea behind VSEPR theory is that electron groups around a central atom will try to get as far away from each other as possible. Think of electrons as being a little bit like magnets with the same pole facing each other; they push each other away. These electron groups can be either the pairs of electrons that form bonds between atoms, or they can be pairs of electrons that are just hanging out on the central atom, not involved in a bond. These unshared pairs are called "lone pairs." So, you know, they all want their own space.
By counting up the number of bonding pairs and lone pairs around the central atom, we can predict the overall arrangement of these electron groups. This arrangement is called the "electron geometry." Once we have that, we can then figure out the "molecular geometry," which is the actual shape formed by the atoms themselves. It’s a two-step process, but it works really well for predicting shapes like the one we see in AlCl3.
The Role of Electron Pairs in Molecular Geometry
Let's talk a bit more about how those electron pairs play a part in determining the molecular geometry, specifically for AlCl3. In the case of aluminum chloride, the central aluminum atom is connected to three chlorine atoms. This means there are three pairs of electrons that are involved in making those connections, or three "bonding pairs." What's really important here, though, is that the aluminum atom in AlCl3 doesn't have any unshared, lone pairs of electrons hanging around on it. That's a key detail.
Because there are three bonding pairs and zero lone pairs around the central aluminum atom, those three bonding pairs spread out as much as they can. The most spacious arrangement for three groups around a central point is a flat triangle. This means that both the electron geometry and the molecular geometry for AlCl3 are trigonal planar. The electron groups are in a flat triangle, and since there are no lone pairs pushing atoms around differently, the atoms themselves also form that same flat triangle.
This kind of arrangement, where everything is flat and spread out, results in bond angles of about 120 degrees between the chlorine atoms. It’s the most balanced way for them to exist, really, minimizing any pushing or pulling between the electron groups. This specific setup helps us understand many of AlCl3's qualities, like whether it tends to pull electrons unevenly or not, which can affect how it reacts with other things. So, you see, those electron pairs are pretty important.
Does AlCl3 Change Its Molecular Geometry?
It's interesting to consider whether a molecule's shape, its molecular geometry, can actually change. For AlCl3, the answer is yes, in a way, depending on its surroundings. While the simple AlCl3 molecule usually takes that flat, triangular shape we've been talking about, aluminum chloride can exist in different forms based on things like temperature and whether it's a solid, a liquid, or a gas. This means its overall arrangement of atoms can shift.
For instance, when aluminum chloride is in its gas phase at higher temperatures, you'll often find it as individual AlCl3 units, each with that trigonal planar shape. But if you cool it down a bit, especially in the gas phase, or if you look at it as a solid, it likes to pair up. It forms something called a dimer, which is written as Al2Cl6. In this dimeric form, the atoms arrange themselves quite differently. Each aluminum atom in the dimer actually becomes surrounded by four chlorine atoms, taking on a shape that's more like a tiny pyramid with a triangular base, or what chemists call a tetrahedral arrangement.
So, while the basic AlCl3 unit has a specific shape, the compound itself can adopt different structures depending on the conditions. This ability to change its arrangement, or its molecular geometry, is a pretty neat feature of aluminum chloride. It shows that even tiny particles can be quite adaptable, you know, responding to their environment.
How to Picture AlCl3's Molecular Geometry?
If you're trying to picture the molecular geometry of AlCl3 in your head, think of it this way. Imagine a single aluminum atom sitting right in the middle. Now, picture three chlorine atoms, each connected to that central aluminum. If you were to lay this whole arrangement down on a table, it would lie perfectly flat. The three chlorine atoms would form the corners of a triangle, and the aluminum would be right in the center of that triangle. It's a very neat and symmetrical setup, really.
This flat, triangular arrangement is what "trigonal planar" means. It's not a pyramid, and it's not bent; it's just a flat shape. The bonds between the aluminum and each chlorine atom are all the same length, and the angles between any two chlorine atoms, as measured from the aluminum, are all equal, about 120 degrees. This equal spacing helps to keep everything stable and balanced. It’s like setting up a tripod; the legs spread out evenly to give the best support, you know?
When you draw out the Lewis structure for AlCl3, you can see how this shape comes about. You'll place the aluminum in the middle, draw single lines connecting it to three chlorine atoms, and then put the remaining electrons around the chlorine atoms. This visual representation, in some respects, really helps to reinforce the idea of that flat, triangular molecular geometry. It’s a very tidy arrangement for such small components.
Why Does AlCl3 Have This Specific Molecular Geometry?
The reason AlCl3 ends up with its particular molecular geometry, that flat, triangular shape, goes back to the basic principles of how atoms like to arrange their electrons. Aluminum, as a central atom, wants to have its outer electrons, the ones involved in bonding, spread out as much as possible. This is all about minimizing the repulsion between those electron groups. Think of it as everyone wanting their personal space; electrons are no different.
In AlCl3, the aluminum atom forms three single bonds with three separate chlorine atoms. There are no extra, unshared pairs of electrons on the aluminum itself. So, you have three distinct groups of electrons around the central atom, all of them bonding pairs. To get as far away from each other as they can, these three groups push each other into a flat, triangular pattern. This arrangement allows for the greatest distance between them, reducing any uncomfortable electron-electron pushing. It’s the most stable way for them to sit, basically.
This fundamental desire for electron groups to spread out evenly is what dictates the shape. If there were, say, four bonding groups, they would arrange themselves into a tetrahedron. If there were two bonding groups, they'd go into a straight line. But for three bonding groups with no lone pairs, the trigonal planar molecular geometry is the natural and most comfortable arrangement for AlCl3. It’s a pretty elegant solution for atomic spacing.
What Makes AlCl3 Nonpolar in Its Larger Form?
Now, let's touch on something else about AlCl3 that's related to its shape: whether it's polar or nonpolar. When we talk about a molecule being polar, it means that the electrons are not shared equally between the atoms, creating a slight positive end and a slight negative end, like a tiny magnet. For the individual AlCl3 molecule in its trigonal planar molecular geometry, the bonds between aluminum and chlorine do have some polarity because chlorine pulls electrons a bit more strongly than aluminum. However, because the molecule is so perfectly symmetrical, with the three chlorine atoms pulling equally in opposite directions in that flat triangle, these individual pulls cancel each other out. So, the individual AlCl3 molecule is considered nonpolar.
But it gets even more interesting when AlCl3 forms its dimeric structure, Al2Cl6. As we mentioned, in this larger form, the atoms arrange themselves in a way that gives each aluminum atom a tetrahedral surrounding, where it's connected to four chlorine atoms. Even though the overall structure is more complex, this dimeric form is also typically considered nonpolar. This is because the overall arrangement of charges within the Al2Cl6 molecule also ends up being very symmetrical. Any slight unevenness in electron sharing within the bonds is balanced out by the molecule's overall shape, meaning there's no net positive or negative end to the whole thing. It's a pretty balanced setup, all things considered.
This idea of polarity, or the lack of it, is very important because it affects how a substance dissolves in different liquids and how it interacts with other molecules. A nonpolar molecule, like AlCl3 in both its single and dimeric forms, tends to dissolve well in nonpolar solvents, like oils, but not so well in polar solvents, like water. It’s a direct consequence of its molecular geometry and the way electron pulls balance out, or don't, within the structure.
- Brian Deegan Net Worth 2024 A Comprehensive Overview
- Livvy Dunne Leaka The Rising Star In The Social Media And Sports World
- Is Frank From American Pickers Alive A Deep Dive Into His Life And Career
- Shiloh Joliepitt News The Life And Journey Of Angelina Jolie And Brad Pitts Daughter
- Are The American Pickers Still Alive A Deep Dive Into Their Journey
Molecular Geometry Structure of Elements Stock Vector - Illustration of

molecular geometry.ppt

PPT - 6-5: Molecular Geometry PowerPoint Presentation, free download