Aluminum Chloride Structure - A Look Inside
Have you ever stopped to think about how something as seemingly simple as a chemical compound actually holds itself together? It's pretty interesting, actually. Take aluminum chloride, which chemists often call AlCl3. This substance, you see, is a common player in many industrial processes, and its basic building blocks, its structure, can really change depending on the conditions it finds itself in. Understanding how its pieces fit together is a pretty big deal for anyone wanting to work with it, or just for someone curious about the tiny bits that make up our world, in a way.
When we picture AlCl3, we might think of a single, neat little unit. But, as a matter of fact, this compound has a bit of a secret life, especially when it gets warm enough to melt or turn into a gas. It doesn't always stay as that simple AlCl3. Instead, it often prefers to pair up, forming something called a dimer. This dimer, known as Al2Cl6, is a much larger arrangement of atoms, and it shows a pretty clever way these elements can connect with each other, you know.
Figuring out these atomic arrangements, like the specific shape and connections within AlCl3 or its dimer, helps us predict how it might act in different situations. It helps us understand why it behaves the way it does when it's helping other chemicals react, or even just how it might dissolve in water. It’s all tied to how those atoms link up, which, you know, is quite something to think about.
- Movierulz Embracing A Sexpositive Culture Through Cinema
- Tia Kemp Birth Chart Unveiling The Mysteries Of Her Astrological Profile
- Cillian Murphy Family A Glimpse Into The Life Of The Acclaimed Actor
- How Old Is Frank Fritz From American Pickers A Comprehensive Look Into His Life And Career
- Dhruv Age Exploring The Rising Stars Journey And Achievements
Table of Contents
- AlCl3 Molecular Structure - A Single Unit?
- What Happens to alcl3 molecular structure When It Heats Up?
- How Do We Draw alcl3 molecular structure?
- Why is alcl3 molecular structure Important for Industry?
- Does alcl3 molecular structure Affect Its Solubility?
- alcl3 molecular structure in Action - Reactions with Other Chemicals
- Figuring Out the Shape of alcl3 molecular structure
- A Quick Look Back at alcl3 molecular structure
AlCl3 Molecular Structure - A Single Unit?
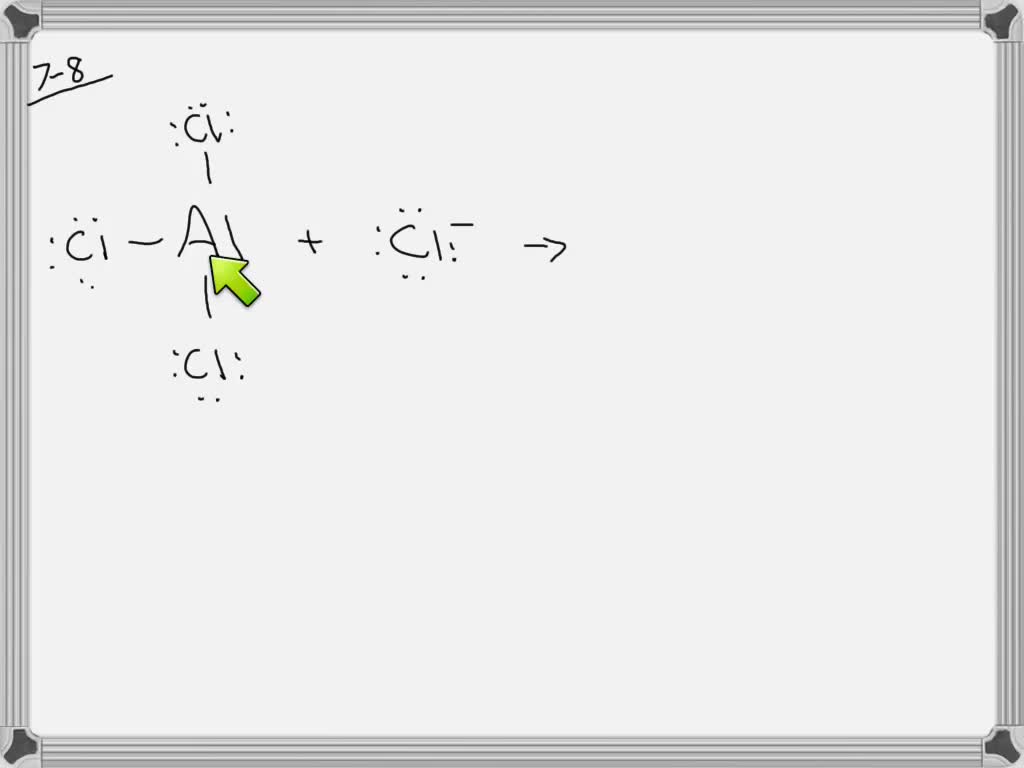
When we first consider aluminum chloride, or AlCl3, we often picture it as one aluminum atom joined up with three chlorine atoms. This is its simplest chemical formula, and it suggests a particular way these atoms might be arranged. For someone trying to figure out how chemicals are put together, the first step often involves sketching out what's called a Lewis structure. This kind of drawing helps us visualize where the electrons are hanging out around each atom and how they connect to form bonds, you know, in a basic way.
To draw the Lewis structure for AlCl3, you would put the aluminum atom right in the middle, since it's typically the atom that forms connections with the most other atoms in this kind of setup. Then, the three chlorine atoms would be arranged around it. Each atom brings its own set of electrons to the table, and these electrons are shared or moved around to create the chemical bonds that hold the whole thing together. It's a way to represent the electron arrangement that makes the compound stable, more or less.
The goal with a Lewis structure is to show how each atom gets a stable number of electrons, often eight for many atoms, which is called an octet. Aluminum, however, is a bit of an exception here. It doesn't always need a full eight electrons around it when it forms bonds. This little difference, actually, is part of what makes AlCl3 behave in some rather unique ways, especially when it's interacting with other chemical compounds. So, it's not always as straightforward as it seems at first glance, is that right?
- How Did Frank Fritz Pass Away A Comprehensive Insight
- Frank Fitz The American Pickers Journey And Legacy
- Jennifer Hudsons Current Boyfriend A Deep Dive Into Her Love Life
- Tia Kemp The Rising Star In The World Of Content Creation
- Exploring The Cinematic Legacy Of Mel Gibson Movies
What Happens to alcl3 molecular structure When It Heats Up?
Here's where the story of aluminum chloride gets a little more involved. While we often think of it as AlCl3, when it's in a melted state or as a gas, it doesn't stay as those individual AlCl3 units. Instead, it transforms into something larger, a paired-up version called Al2Cl6. This bigger structure is known as a dimer. It's like two of the smaller AlCl3 pieces decide to link arms and form a single, bigger molecule. This change in its alcl3 molecular structure is a really key thing to keep in mind, too, when you're working with it at higher temperatures.
In each of these Al2Cl6 molecules, the way the atoms connect is pretty clever. You have two aluminum atoms, and they are joined together by two chlorine atoms that act as bridges. It's almost like these two chlorine atoms are holding hands with both aluminum atoms at the same time, creating a stable connection between the two AlCl3 units that originally came together. This bridging arrangement is a common way for atoms to connect in chemistry, and it's something you see in other compounds as well, just like two hydrogen atoms might bridge between other atoms in a different chemical setup.
This shift from a single AlCl3 unit to the Al2Cl6 dimer is quite important for how the substance acts. Its alcl3 molecular structure in the gas phase, for instance, means it has a different shape and size compared to what you might expect if it stayed as just AlCl3. This has implications for things like its boiling point and how it moves around as a gas. It's a clear example of how temperature can really influence the actual form a chemical takes, which, you know, is pretty neat.
How Do We Draw alcl3 molecular structure?
When we talk about drawing the alcl3 molecular structure, especially in its simple AlCl3 form, we're trying to figure out how all the electrons are arranged around the central aluminum atom. This isn't just about drawing lines for bonds; it's also about understanding the "electron domain geometry" and the "molecular geometry" of that central atom. These terms sound a bit technical, but they basically describe the shape that the electrons and the atoms around the central aluminum atom take on, so.
First, for the electron domain geometry, you'd count all the places where electrons are found around the aluminum atom. This includes any bonds it forms with chlorine atoms and any lone pairs of electrons that might be sitting on the aluminum itself. In the case of AlCl3, the aluminum atom usually has three bonds to chlorine atoms and no lone pairs. This arrangement, with three electron "groups" around the center, typically leads to a shape where those groups try to get as far away from each other as possible, which, you know, makes a triangular flat shape.
Then, for the molecular geometry, you only look at the actual atoms, ignoring any lone pairs of electrons on the central atom. Since AlCl3 has three chlorine atoms connected to the central aluminum and no lone pairs on the aluminum, the actual shape of the molecule itself also ends up being that flat triangular arrangement. This particular alcl3 molecular structure, with its atoms spread out in a flat triangle, helps explain some of its chemical behavior, like how it might interact with other molecules that have a certain shape, you know, when they get close.
It's a way of visualizing the three-dimensional arrangement, which is really helpful for chemists. By drawing these structures and figuring out these geometries, we get a much better sense of how the molecule will sit in space and how it might bump into or connect with other molecules during a reaction. It's a fundamental step in understanding how chemicals work, basically.
Why is alcl3 molecular structure Important for Industry?
Aluminum chloride, or AlCl3, plays a very big part as a catalyst in many different industrial reactions. A catalyst, you see, is something that helps a chemical reaction happen faster or more easily without actually being used up itself. It's like a helpful assistant that speeds things along. The specific alcl3 molecular structure, whether it's the single AlCl3 unit or the Al2Cl6 dimer, is really important for its ability to act as this kind of helper in these big industrial processes, in some respects.
For instance, one of the ways aluminum chloride is often prepared is from chloride gas. This preparation method ensures that the AlCl3 is ready to do its job. Its particular structure allows it to accept electron pairs from other molecules, which is a key step in many of the reactions it helps to speed up. This ability to accept electrons is what makes it a strong Lewis acid, a type of chemical that is very good at promoting certain kinds of reactions, which, you know, is quite useful.
The fact that AlCl3 can exist in different forms, like the dimer in the gas phase, means that its effectiveness as a catalyst can change depending on the temperature and pressure of the industrial process. Engineers and chemists need to understand these structural changes to make sure the reactions run as smoothly and efficiently as possible. So, knowing about the alcl3 molecular structure isn't just for academic curiosity; it has very real, practical applications in making all sorts of products we use every day, you know, in a practical sense.
Does alcl3 molecular structure Affect Its Solubility?
When we talk about how much of a substance can dissolve in a liquid, like water, we're talking about its solubility. For aluminum chloride, or AlCl3, its solubility is known to be about 45.8 grams for every 100 milliliters of solution at a temperature of 20 degrees Celsius. This number tells us how much AlCl3 you can pack into a certain amount of water before it stops dissolving and just sits at the bottom. The alcl3 molecular structure, and how it interacts with water molecules, is a big part of why it dissolves to this particular extent, too.
Beyond simple solubility, chemists sometimes look at something called the Ksp, or solubility product constant, for compounds that don't dissolve completely but rather break apart into ions when they hit the water. While the provided information gives us the solubility of AlCl3, figuring out its Ksp would involve looking at how it separates into aluminum ions and chloride ions in solution. This calculation also needs the molar mass of AlCl3, which is about 133.34 grams for every unit. This figure, you know, helps convert between the amount of substance and its weight.
The way the alcl3 molecular structure interacts with water molecules is pretty interesting. When AlCl3 dissolves, it doesn't just disappear; it typically breaks down into its charged parts, the aluminum ion and the chloride ions. These charged pieces then get surrounded by water molecules. The strength of the bonds within the AlCl3 molecule and how easily those bonds can be pulled apart by water molecules are all tied to its structure. So, the way it's built really does influence how it behaves when you try to dissolve it, basically.
alcl3 molecular structure in Action - Reactions with Other Chemicals
The alcl3 molecular structure plays a really important part in how it helps other chemicals react with each other. It's often used to encourage specific types of organic reactions, which are reactions involving compounds that contain carbon. For instance, if you take a lot of benzene, which is a particular kind of ring-shaped carbon compound, and mix it with dichloromethane, all in the presence of AlCl3, you'll get a new product. AlCl3 acts as the catalyst here, guiding the reaction to create something different, you know, a new compound.
Similarly, AlCl3 can help predict what major organic product will form when certain compounds mix together. It has a knack for steering reactions in a particular direction. The way the alcl3 molecular structure is set up allows it to act as that electron-pair acceptor we talked about earlier, which is a key step in many of these organic transformations. It helps to activate other molecules, making them more ready to react, which, you know, is quite a trick.
Another example involves a compound called aniline reacting with methyl chloride, or CH3Cl, again with AlCl3 present. In this reaction, AlCl3 helps the methyl group from CH3Cl attach to the aniline molecule. When you draw the structure of the product from this reaction, you'd need to make sure you show any formal charges, which are like imaginary charges assigned to atoms in a molecule, and the correct number of hydrogen atoms attached to the nitrogen atom. All of this depends on how the alcl3 molecular structure guides the process, basically, in a very specific way.
Figuring Out the Shape of alcl3 molecular structure
When we talk about the shape of the alcl3 molecular structure, we're trying to figure out how the atoms arrange themselves in three-dimensional space. This isn't just a random arrangement; atoms try to position themselves to minimize repulsion between their electron clouds. For a molecule like AlCl3, once you've drawn its Lewis structure, the next step is to figure out its electron domain geometry and its molecular geometry around the central aluminum atom. This helps us visualize its actual shape, you know, in a practical sense.
The electron domain geometry looks at all the areas where electrons are located around the central atom, whether they are in bonds or in lone pairs. For AlCl3, with its central aluminum atom, there are three areas of electron density, since it forms three bonds with chlorine atoms and doesn't have any unshared electron pairs on the aluminum itself. These three areas will spread out as much as they can to reduce any pushing away from each other, which means they will form a flat, triangular shape, with the aluminum in the very middle and the chlorines at the corners, very evenly spaced.
Then, the molecular geometry considers only the positions of the atoms themselves, not the lone pairs. Since there are no lone pairs on the central aluminum atom in AlCl3, the molecular geometry ends up being the same as the electron domain geometry: a flat triangle. This specific alcl3 molecular structure, with its atoms arranged in a trigonal planar shape, is important for how it interacts with other molecules. It's like having a specific key for a specific lock; the shape matters a great deal for how chemicals fit together and react, basically.
Understanding these geometries is a fundamental part of chemistry. It helps us predict how a molecule might behave, how strong its bonds might be, and even how it might stack up with other molecules in a solid form. It's all connected to the way the electrons and atoms arrange themselves in space, which, you know, is pretty cool.
A Quick Look Back at alcl3 molecular structure
So, we've taken a little walk through the world of aluminum chloride, focusing on its alcl3 molecular structure. We've seen how this compound, often written simply as AlCl3, can actually exist in a more complex, paired-up form called Al2Cl6, especially when it's hot enough to be a liquid or a gas. This pairing involves clever bridging chlorine atoms that hold the two aluminum atoms together, you know, in a strong connection.
We also touched on how we figure out the shape of these molecules, using things like Lewis structures to map out electrons and then determining the electron domain and molecular geometries around the central aluminum atom. This helps us see that the simple AlCl3 unit often takes on a flat, triangular shape. This shape, and the ability of AlCl3 to change its form, is very important for its job as a catalyst, speeding up many different reactions in industry, in some respects.
Beyond its role as a reaction helper, we also considered how its structure influences things like its solubility in water and how it might break apart into ions. And, we looked at how AlCl3 is used to help make new compounds in organic chemistry, guiding reactions involving things like benzene and aniline. All these different aspects, actually, tie back to the specific way the atoms in aluminum chloride are arranged and how that alcl3 molecular structure can shift depending on its surroundings, so.
- Exploring The World Of America Pickers A Deep Dive Into Antique Treasures
- Frank Fritz Of American Pickers The Journey Of A Treasure Hunter
- Moveruls A Deep Dive Into The Phenomenon Of Modern Moving Services
- What Is Ozzy Osbournes Net Worth An Indepth Analysis
- Exploring The Life And Career Of Frank Frit A Comprehensive Biography

Alcl3 Lewis Structure (Aluminum Chloride), 45% OFF

Aluminium Chloride Alcl3 Molecule Simple Molecular Stock Vector
SOLVED:Aluminum chloride, AlCl3, behaves more as a molecular compound