AlCl3 Geometry - Understanding Its Unique Shapes
Have you ever wondered how tiny bits of matter, like molecules, actually look? It's kind of fascinating, you know, to think about the invisible world around us and how everything fits together. Well, when we talk about things like aluminum chloride, or AlCl3, its shape is a pretty big deal. This particular compound shows off some rather interesting arrangements, depending on its surroundings, and it’s something chemists find quite intriguing.
So, we're going to take a closer look at what makes AlCl3 stand out in the chemical crowd. It’s not just a simple case of one fixed shape; actually, it changes its form quite a bit. This ability to switch shapes helps us understand why it acts the way it does in different situations, whether it's floating around as a single unit or linking up with another one. It's almost like it has a few different outfits it can wear, depending on where it's going.
Basically, getting a good grasp of the shape of AlCl3 is really helpful for anyone trying to figure out how it might react with other chemicals. This knowledge, you see, helps people in science and industry predict what will happen when they mix things together. It's all about figuring out the puzzle pieces of the chemical world, and the shape of AlCl3 is a key piece in many of those puzzles, just a little bit important.
- Did Frank Fritz Of American Pickers Pass Away
- Frank Frizt
- Jennifer Hudson Boyfriend A Deep Dive Into Her Romantic Life
- Understanding The Impact Of Steve Harveys Death A Tribute To His Legacy
- Shawn Kyle Swayze The Rise And Legacy Of A Modern Icon
Table of Contents
- What is the Basic AlCl3 Geometry Like?
- The AlCl3 Geometry of a Single Molecule
- How Does AlCl3 Geometry Change?
- The Dimer AlCl3 Geometry - A Pair
- What Makes AlCl3 Geometry Unique?
- How Does AlCl3 Geometry Affect its Behavior?
- Why is AlCl3 Geometry Important?
- AlCl3 Geometry in Solids
What is the Basic AlCl3 Geometry Like?
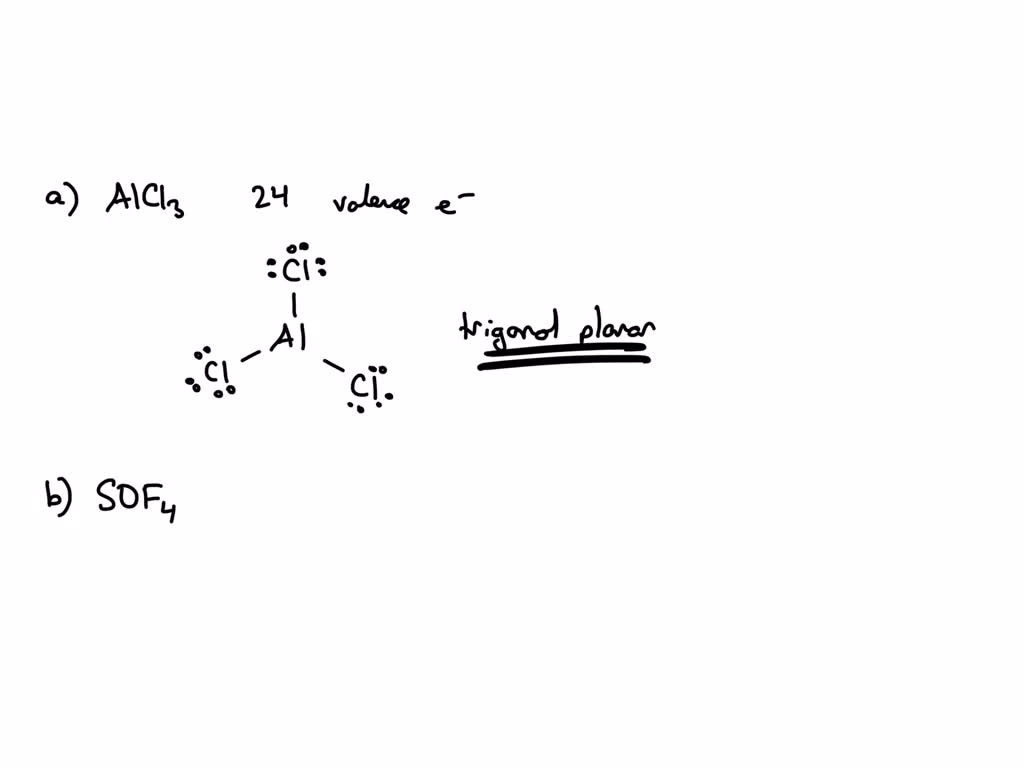
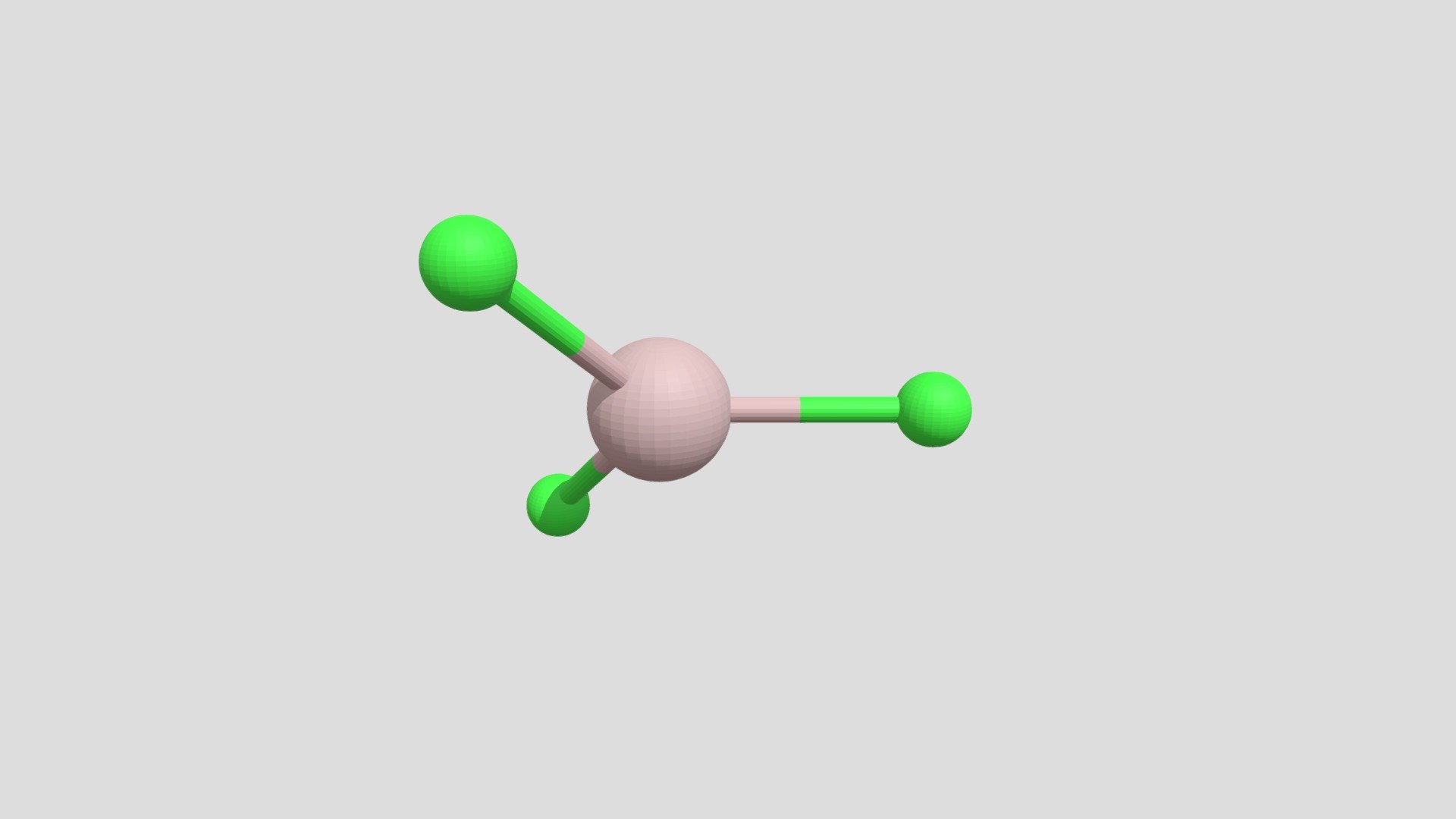
When you first meet aluminum chloride as a single, separate molecule, its shape is pretty straightforward. You have one aluminum atom sitting right in the middle, and then three chlorine atoms are spread out evenly around it. Think of it like a flat triangle, with the aluminum in the very center and the chlorines at each point. This arrangement is quite common for molecules with three atoms connected to a central one, especially when there are no extra unshared electron pairs hanging around on the central atom. It's a very neat and tidy setup, you know, for a molecule.
This particular shape, often called "trigonal planar," means all four atoms lie on the same flat surface. The angles between the bonds, the lines connecting the aluminum to each chlorine, are all equal, measuring about 120 degrees. So, it's a very balanced and symmetrical shape, which helps explain some of its qualities. It's basically the simplest way these atoms can arrange themselves while keeping everything as far apart as possible, given the number of things connected to the center, or something like that.
The AlCl3 Geometry of a Single Molecule
To get a bit more specific about the AlCl3 geometry when it's just one molecule, we look at the electron arrangements. Aluminum, being in group 13 of the periodic table, usually likes to make three connections. In AlCl3, it forms three single connections with three different chlorine atoms. There are no lone pairs of electrons left over on the aluminum atom, which is a key detail. This lack of extra electron pairs is what pushes the three chlorine atoms into that flat, triangular shape, sort of like spokes on a wheel.
- Sza Age Everything You Need To Know About The Rising Star
- Sophia Rain The Rising Star Of Ero Me
- Adnan Syeds Wife A Journey Through Love And Loyalty
- Exploring The Life And Legacy Of Law Aubreigh Wyatt
- Tia Kemp Net Worth 2024 A Comprehensive Analysis
This particular arrangement is what gives the molecule its specific properties when it's in a gas state, for instance, where it can float around freely as individual units. It's a very efficient way for the atoms to be positioned, minimizing any pushing or pulling between the electron clouds around each atom. Basically, it’s all about finding the most comfortable spot for everyone involved, you know, in the molecule's structure.
How Does AlCl3 Geometry Change?
Now, here's where it gets really interesting with AlCl3 geometry. While that flat triangle shape is true for a single molecule, aluminum chloride doesn't always stay as just one molecule. When it's in a gas state at lower temperatures, or even when it's a liquid, it tends to pair up. This pairing creates a completely different structure, something called a "dimer." It's like two of those triangular pieces decide to hold hands and form a new, bigger shape. This transformation is a pretty cool trick it pulls, actually, making its shape quite adaptable.
This change happens because the aluminum atom, in its original flat triangular form, is a bit "electron deficient." It doesn't quite have a full set of electrons around it, making it eager to grab more. So, it reaches out to another AlCl3 molecule, using some of the chlorine atoms as bridges. This linking up changes the geometry around each aluminum atom quite dramatically, moving it away from that simple flat triangle. It's almost like a small building block finding a way to connect with another identical block, forming a larger, more stable structure, you see.
The Dimer AlCl3 Geometry - A Pair
When two AlCl3 molecules join together to form a dimer, the geometry around each aluminum atom shifts from being flat and triangular to something more like a pyramid with a triangular base. This new shape is often called "tetrahedral." In this dimer, two chlorine atoms act as bridges, connecting the two aluminum atoms. Each aluminum atom is now connected to four chlorine atoms: two that are just connected to that aluminum, and two that are shared with the other aluminum. This arrangement makes for a much more crowded, yet stable, setup.
Imagine two of those original flat triangles tilting and merging at two of their points. The shared chlorine atoms become the "hinges" of this new structure. This change to a tetrahedral arrangement around each aluminum atom is a direct result of the aluminum trying to get a full set of eight electrons around itself, which it achieves by sharing with the other molecule. It's a clever way for the molecule to become more stable, basically, by teaming up.
What Makes AlCl3 Geometry Unique?
What really makes AlCl3 geometry stand out is this ability to switch between shapes based on its environment. Many molecules have one fixed shape, and that's that. But AlCl3, it shows off a kind of flexibility. This isn't just a random happening; it's deeply tied to how aluminum atoms behave and their tendency to try and complete their electron shells. This adaptability in its form makes it a particularly interesting subject for study, you know, in chemistry classes.
The transition from a simple trigonal planar shape to a more complex tetrahedral arrangement in the dimer form highlights a fundamental principle in chemistry: molecules will often change their structure to achieve a more stable state. For AlCl3, this means getting more electrons around its central aluminum atom. This kind of shape-shifting isn't something you see every day with every compound, which is why AlCl3 is often pointed out as a good example of this behavior, or something like that.
How Does AlCl3 Geometry Affect its Behavior?
The changing AlCl3 geometry has a big impact on how it acts in different situations. For instance, its ability to form a dimer affects its boiling point and melting point. The dimer, being a larger and more complex structure, requires more energy to break apart, meaning it will typically have a higher boiling point than if it just stayed as individual, smaller molecules. This structural change also plays a role in how it dissolves in different liquids and how it reacts with other substances. It's a pretty big deal, actually, for its overall performance.
When AlCl3 acts as a "Lewis acid," meaning it likes to accept electron pairs, its trigonal planar shape is perfect for this. The empty space around the aluminum atom makes it very eager to welcome new electron pairs. When it forms the dimer, it's essentially satisfying that electron hunger within itself. This shape-behavior link is a really important concept in understanding chemical reactions, so, it's worth paying attention to.
Why is AlCl3 Geometry Important?
Understanding the AlCl3 geometry isn't just for academic curiosity; it has real-world importance. Aluminum chloride is used in many industrial processes, like making other chemicals, or as a catalyst to speed up reactions. Its ability to switch shapes and its electron-accepting nature are key to its usefulness in these applications. If you didn't know its shape, you couldn't really predict how it would behave or how to best use it, you know, in a practical sense.
For example, in organic chemistry, AlCl3 is a common tool in reactions where new carbon-carbon bonds are formed. Its specific geometry, especially its ability to accept electron pairs, is what allows it to kickstart these reactions. So, basically, knowing about its shape helps scientists and engineers design better processes and create new materials. It's a pretty fundamental piece of information, you see, for working with this compound.
AlCl3 Geometry in Solids
When AlCl3 is in its solid form, the arrangement of atoms becomes even more extended and intricate. It doesn't just stay as individual molecules or simple dimers. Instead, it forms a large, continuous network. In this solid structure, each aluminum atom is surrounded by six chlorine atoms, creating an octahedral arrangement. This is a very different setup from the flat triangle or the tetrahedral dimer, and it shows just how much the AlCl3 geometry can change depending on its physical state. It's almost like a building that can be assembled in many different ways, depending on the space available.
This solid structure is made up of layers of aluminum and chlorine atoms, all connected in a repeating pattern. The way these atoms are packed together in the solid state gives AlCl3 its specific physical properties as a solid, like its melting point and how it conducts electricity, if at all. So, the shape is still a big player, even when it's all packed together tightly. It’s a pretty good example of how structure influences properties, you know, across different states of matter.
So, to recap, the shape of aluminum chloride, or AlCl3 geometry, is a really interesting topic because it's not just one fixed thing. It can be a flat triangle when it's a single molecule floating around. Then, it can team up with another molecule to form a pair, where each aluminum atom takes on a pyramid-like shape. And when it's a solid, it forms a much larger, interconnected structure where each aluminum is surrounded by six chlorines. These different shapes are important because they tell us a lot about how AlCl3 acts and why it's useful in various ways.
- Understanding Robert Sapolsky A Comprehensive Insight Into The Life And Work Of A Renowned Neuroscientist
- Is Mike Wolfe Dead The Truth Behind The Rumors
- How Old Is Tia Kemp Discovering The Life And Age Of This Influential Figure
- Did Frank Fritz Of American Pickers Pass Away
- Exploring The Life And Legacy Of Chris Burke A Journey Through Challenges And Triumphs

Alcl3 Lewis Structure Geometry Hybridization And Polarity | itechguides
SOLVED: Draw the Lewis structure for each of the following and THEN
Alcl3 Molecular Geometry